Research Programme: Dissecting the role of WWP1 in cancer
Principal Investigator: Prof. Francesca Bernassola

An Overview of her Scientific Interests
During and after her training, Dr Bernassola has focused her scientific interests on the functional characterization of the two p53 homologues, p63 and p73. Her efforts have been primarily directed towards studying the molecular mechanisms underlying the regulation of p63 and p73 as well as their biological functions, with major emphasis on their cancer-related activities. Specifically, Dr Bernassola’s group sought to identify and characterize ubiquitin-dependent pathways controlling p73 and p63 protein stability and transcriptional function. These studies contributed to unveil the regulation of p73 and p63 by the tumour suppressor promyelocytic leukemia protein PML (Bernassola F, et al., J Exp Med. 2004; Bernassola et al., Oncogene 2005). More recently, her studies identified p63 as a crucial regulator of mammary cancer stem cell biology (Memmi et al., Proc Natl Acad Sci U S A, 2014). Dr Bernassola has also been actively working at identifying and characterizing the role of HECT-type ubiquitin ligases in cancer development and progression (Oberst et al., Proc Natl Acad Sci U S A. 2007; Bernassola et al., 2008; Sanarico et al., Leukemia 2018; Bernassola et al., Trends Biochem Sci. 2019).
Ongoing Research Projects
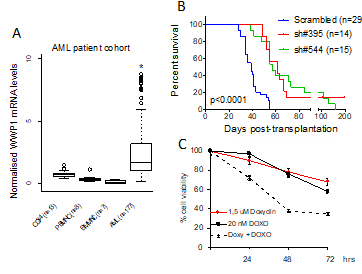
The E3 ubiquitin ligase WWP1 is frequently amplified and overexpressed in several cancers, including acute myeloid leukemia (AML), in which it acts as oncogenic factor by promoting cell proliferation and survival. Deregulated expression of WWP1 has been associated with unfavourable prognosis. WWP1 inactivation supresses cancer cell growth and sensitizes cancer cells to chemotherapy-induced apoptosis. Nevertheless, the molecular basis underlying the ability of WWP1 to promote leukemogenesis and to influence the effectiveness of chemotherapy remains largely unknown. At present, the major goals of Dr Bernassola’s research activity are to characterize the involvement of WWP1 in the pathogenesis and chemoresistance of AML cells, and to identify the molecular targets that mediate its oncogenic functions in leukaemic cells.
The research project will be accomplished through two specific Tasks:
Task 1. Identification of novel WWP1 protein substrates mediating its oncogenic activities in AML cells. Within this task, we foresee to establish the oncogenic potential of WWP1 in AML by dissecting its molecular targets in leukaemogenesis. To reach this goal, it has been exploited a ubiquitin remnant-profiling approach that relies on the immunopurification of ubiquititinated species coupled to mass spectromety. As a part of this Task, we will biochemically and functionally validate LC3B and DNA-PKcs as effector targets of WWP1 in the regulation of autophagy and chemo responsiveness, respectively.
Task 2. Effect of WWP1 inhibition on leukaemia growth and chemotherapy sensitivity in vivo. To investigate the oncogenic role of WWP1 in vivo, we will exploit genetic inactivation and pharmacological inhibition of WWP1 in several preclinical mouse and human models of leukaemogenesis. In particular, we seek to test whether WWP1 influences chemosensitivity of AML through DNA-PKcs regulation.
RELEVANCE FOR CANCER
Despite a high rate of complete remission after cytotoxic agent administration, most AML patients undergo relapse. Thus, there is urgent need to identify novel molecular determinants to improve risk stratification and therapeutic strategies. Testing the effects of inhibitor compounds of WWP1 E3 ligase activity on AML progression and drug resistance will potentially benefit to the development of novel therapeutic strategies to increase the chemoresponsiveness of AML cells.