Research Programme: Dissecting the Role of ZNF750 in Cancer
Principal Investigator: Prof. Massimiliano Agostini

Scientific Interest
The p53 family (p53/p63/p73) plays relevant roles in cancer and development. Mutations or aberration of the expression of these genes is observed in the vast majority of human cancers as well as in genetic human conditions. Through a complex network of transcriptional targets, including coding and non-coding genes, the p53 family influences a variety of biological processes, including cell death and proliferation, genomic stability, metabolism and stemness. By employing the isoform-selective knockout mouse models of p73, Prof. Agostini demonstrated that p73 is a positive regulator of renewal capacity of neuronal stem cells. He also characterized the molecular pathways underlying the role of this gene in neuronal differentiation, identifing the microRNA-34 as a transcriptional target of p73, suggesting that the axis p73/miR-34a/Synaptotagmin-1 may play a role in the pathogenesis of Alzheimer’s disease. Part of his work addressed the role of p73 in the regulation of energy metabolism using both in vivo and in vitro models.
Current Research
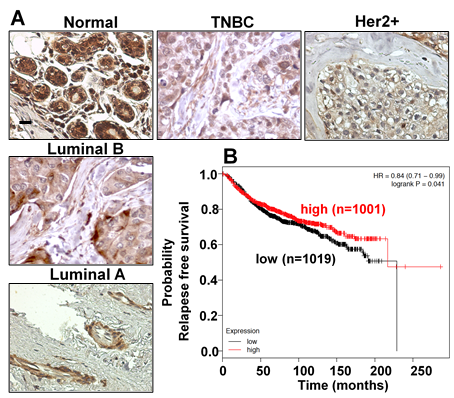
Understanding the molecular mechanism that regulates epithelial homeostasis is essential not only to determine the basis of development and regeneration of organs and tissues, but also to clarify the basis of cancer. ZNF750 is a zinc finger transcription factor that plays a key role in the regulation of the homeostasis of epithelial tissue. Mutations in ZNF750 have been reported in squamous cell carcinomas (SCCs) of the oesophagus, lung and cervix and its function has been consistently associated to tumour suppressive roles, including inhibition of cell proliferation and migration. By using a bioinformatics approach we indicated that the ZNF750 expression is generally downregulated in breast cancer patients and this deregulation is of prognostic value. Mechanistically, we unveiled that ZNF750 represses breast cancer invasion via epigenetic control of pro-metastatic genes.
We are interested in the investigation of the role of the transcription factor ZNF750 in cancer by using both in vitro and in vivo models combined with systems biology (transcriptomics, proteomics and metabolomics). In particular:
Task 1: Dissection of the transcriptional network
underlying ZNF750 function in oncosuppression
Task 2: Characterisation
of the oncological phenotype of ZNF750 genetically modified mice
Task 3: Correlation of ZNF750 targets/interactors in clinical settings
Relevance to cancer biology
We expect to determine the contribution of ZNF750 protein in tumorigenesis and unveil the underlying molecular mechanisms. The dissection of the network of interactors/regulators/targets of ZNF750 could provide novel therapeutic targets and biomarkers for cancer diagnosis, prognosis. This information may also offer novel options for alternative approaches to enhance the therapeutic response to standard therapies.